
Development
Clinical Development Phases
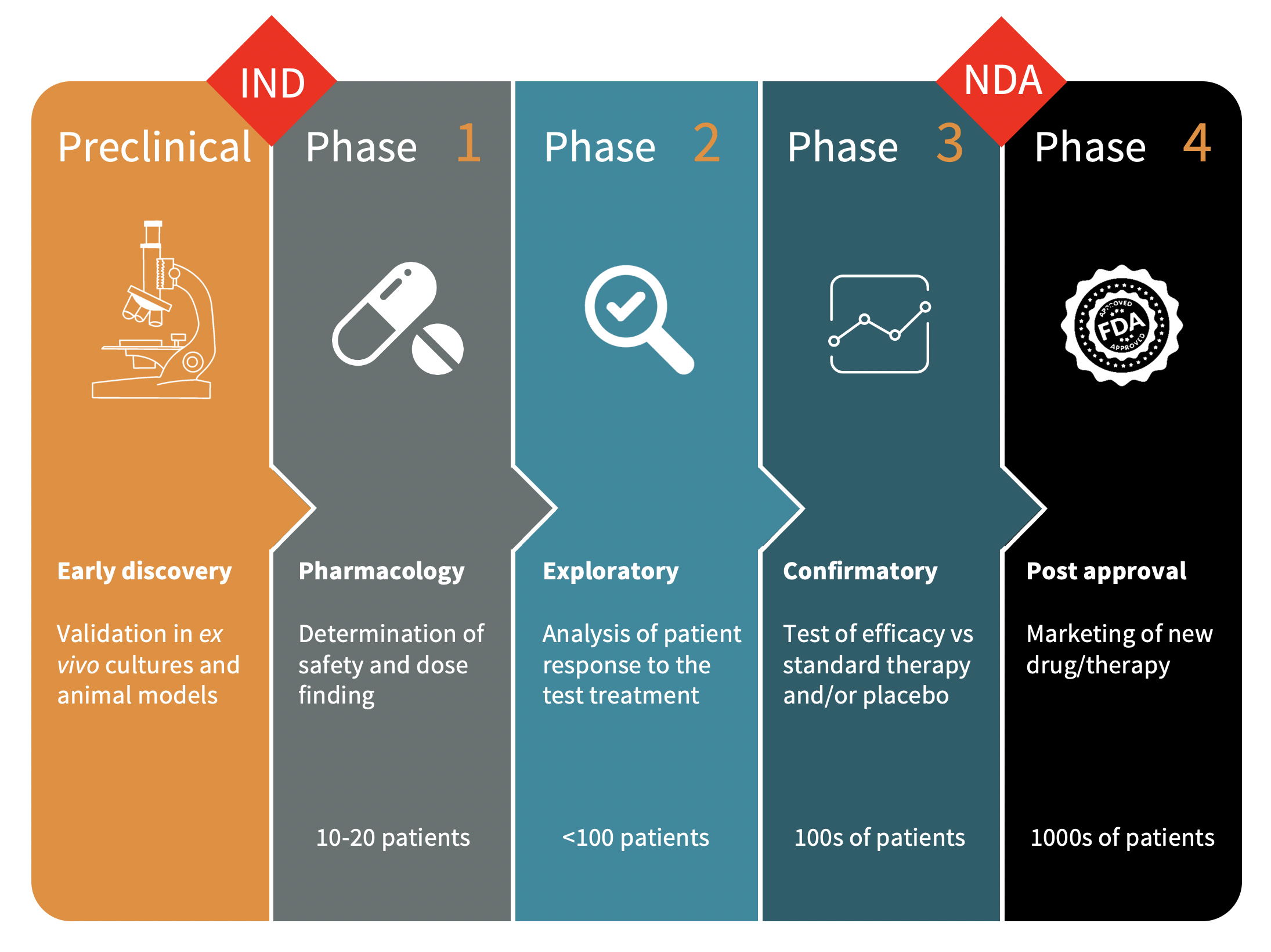
IND: Investigational New Drug NDA: New Drug Approval
Modiblast Plan
In this phase we developed and evaluated multiple drug candidate combinations ex vivo with whole blood samples from AML patients and in an animal model (rats with experimentally induced leukemia).
The most promising candidate (Kit M) was chosen for further research and development. These data were published in numerous peer-reviewed papers.
We aim to treat a cohort of relapsed/refractory AML and MDS patients who are left with little to no options except palliative treatment. The focus is on safety and dose finding. Treatment efficacy shall be evaluated by determining the disease control rate (DCR).
This study is in an advanced planning phase.
The study will seamlessly be extended to a Phase IIa part geared to statistical validation of the efficacy of the immunotherapeutic treatment.
This multi-center POC study may be completed in as little as three years. Recruitment can be accelerated by increasing the number of participating clinics to more than four.
The aim is to reformulate the drug combination to achieve a less invasive and burdensome administration. The equivalence in safety and efficacy as well as the optimal dosage are to be evaluated in a pivotal study.
In parallel, an application for orphan drug status is to be submitted.
